Understanding the difference between natural zeolite and synthetic zeolite
Zeolite, as a crucial mineral, plays a significant role in both industrial and scientific research fields, mainly categorized
into two forms: synthetic zeolite and natural zeolite. Although both are widely used in their respective domains, they
exhibit significant differences in characteristics and advantages.
1. Composition and Structure:
Synthetic Zeolite: Manufactured through chemical synthesis, its composition and structure can be precisely controlled
according to specific requirements, providing it with high flexibility in various application scenarios. The pore structure
and chemical composition of synthetic zeolite can be customized and adjusted based on specific needs.
Natural Zeolite: Being a mineral naturally formed in the Earth's crust, its composition and structure are constrained by
geological conditions, resulting in limited control and potential variations in properties across different geographical locations and time periods.
2. Purification Efficiency:
Synthetic Zeolite: Typically possesses higher adsorption capacity and finds extensive applications in water treatment, gas
adsorption, and catalytic reactions. Due to its adjustable pore structure, synthetic zeolite can better cope with various
environmental conditions and pollutants.
Natural Zeolite: While it exhibits some effectiveness in adsorbing pollutants, its overall performance is inferior to
synthetic zeolite. Its adsorption capacity is limited by its natural state and lacks the adjustability characteristic of synthetic
zeolite.

3. Sustainability:
Synthetic Zeolite: The production process often involves energy consumption and waste emissions, potentially causing
negative environmental impacts. However, some manufacturing methods have focused on reducing environmental effects,
such as using renewable energy and green chemistry techniques.
Natural Zeolite: The extraction and extraction processes may also have environmental impacts, especially in cases of
unregulated extraction. However, its naturally occurring properties help mitigate some environmental issues associated
with the manufacturing process.
4. Cost:
Synthetic Zeolite: The preparation process requires strict control of chemical reactions, resulting in relatively higher costs.
However, in specific applications, its superior performance can offset the cost disadvantage.
Natural Zeolite: It has lower costs since it does not require complex preparation processes. This characteristic makes
natural zeolite more price competitive in large-scale applications.
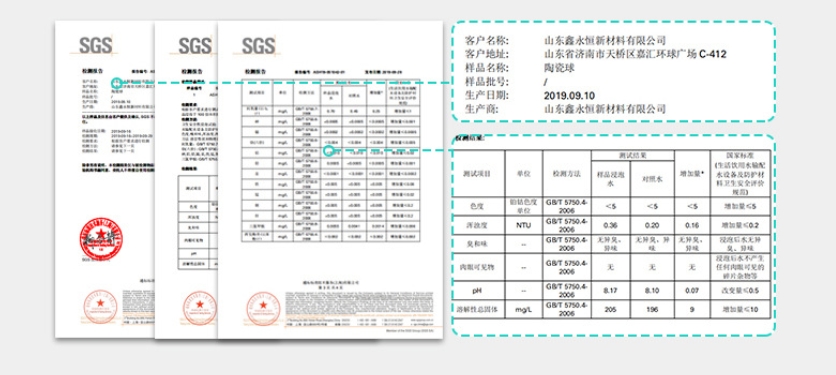
Natural zeolites come in various types, such as mordenite and clinoptilolite, primarily containing calcium and sodium
elements. After activation through special processes, natural zeolites have a lower cost compared to activated carbon.
They are ideal filter materials for industrial water and wastewater treatment, effectively removing trace pollutants from
tap water. Synthetic zeolites, also known as artificial zeolites, are formed by melting a mixture of raw materials such as
sodium carbonate, potassium hydroxide, feldspar, and kaolin into irregular structures. They are commonly used for water
softening, seawater desalination, and pure water production, offering similar functions to natural zeolites.
Traditional methods for synthesizing synthetic zeolites include the water glass method, activated clay method, bentonite
method, kaolin method, and coal gangue method. Among these, the water glass method is mature and easy to control
but has higher costs. The activated clay method and bentonite method require the addition of aluminum sources,
increasing costs and necessitating anti-corrosion treatment for equipment. The kaolin method and coal gangue method
utilize raw materials with aluminum-to-silicon ratios similar to 4A zeolite. They involve a hydrothermal crystallization
reaction in a sodium hydroxide solution, requiring high-temperature roasting of the ore, leading to high energy
consumption and certain environmental pollution.
Synthetic zeolites exhibit superior adsorption performance compared to natural zeolites, with better ion exchange
capabilities. They can remove turbidity, color, and odor from water and also adsorb and exchange harmful heavy metals.
When evaluating the adsorption performance of natural and synthetic zeolites, it's essential to consider various factors,
including their physical and chemical properties, preparation processes, purity, structure, and specific application conditions.
Natural zeolites: Adsorption performance may fluctuate due to differences in origin and zeolite type, stemming from
variations in structure and chemical properties caused by differences in deposition environments and compositions.
Natural zeolites may contain impurities that could affect their adsorption performance.
Synthetic zeolites: Their advantage lies in precise control over chemical composition and structure, allowing customization
according to specific adsorption requirements. Synthetic zeolites typically have higher purity, meaning fewer impurities
interfere, providing more stable and predictable adsorption performance.
In a comprehensive comparison, synthetic zeolites often exhibit superior adsorption performance in specific application
contexts due to their customizability and higher purity, especially for efficient adsorption of specific compounds. However,
for some general adsorption applications, natural zeolites are effective enough and have lower costs.
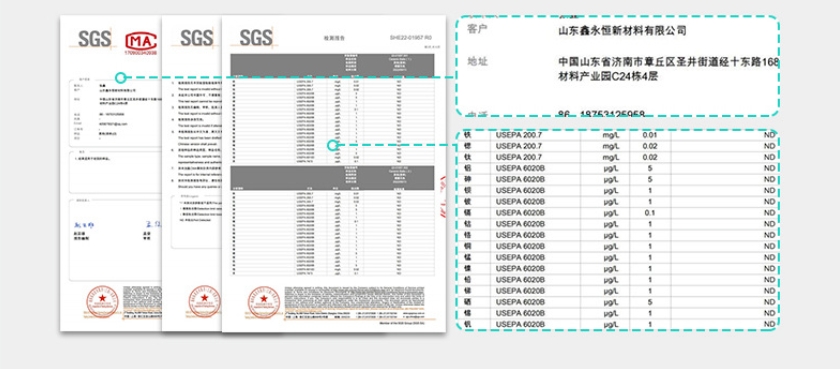
Determining the strength of adsorption performance between natural and synthetic zeolites doesn't have an absolute
answer; it depends on specific application requirements and the desired zeolite characteristics. In highly specialized
applications, synthetic zeolites may have an advantage, while in cost-sensitive or widely applicable scenarios, natural
zeolites may be a more suitable choice.
Natural zeolites are solids with a well-defined three-dimensional microporous crystal structure, containing aluminum,
silicon, and oxygen, with cations and water distributed within the pores. Silicon and aluminum atoms form tetrahedral
coordination by sharing oxygen atoms. Compositionally, zeolites are similar to clay minerals, both being aluminosilicates.
However, they have significant differences in crystal structure: most clays have a layered structure (similar to stacked
playing cards), which undergo volume contraction and expansion upon water absorption and evaporation. Zeolites,
on the other hand, have a rigid three-dimensional honeycomb structure composed of interconnected tunnels and
cage-like networks. Water molecules can freely enter and exit these pores, while the zeolite framework remains rigid.
Another unique feature of this structure is that the pore and channel sizes are nearly uniform, giving the crystal molecular
sieve functionality.
There is a wide variety of zeolite types, with natural zeolite deposits found in many places globally, although most
commercial applications involve synthetic products. It's worth noting that not all zeolite minerals have the same properties,
and there are approximately 50 known types of zeolites (such as clinoptilolite, chabazite, mordenite, etc.), each with
differences in physical and chemical properties, primarily in crystal structure and chemical composition. Additionally,
characteristics such as particle density, cation selectivity, molecular pore size, and strength can vary with zeolite type.
Understanding the specific type of zeolite used is crucial to ensure it meets the practical application requirements.

The pore size of zeolites in the market generally ranges from approximately 3 Å to 8 Å. Common commercial varieties
include Type A, Type β, mordenite, Type Y, and ZSM-5.
The main differences between natural and synthetic zeolites are as follows:
Synthetic zeolites are manufactured through energy-intensive chemical synthesis methods, whereas natural zeolites are
sourced from processing natural mineral deposits. The SiO₂/Al₂O₃ ratio in synthetic zeolites is typically 1:1, whereas in
natural clinoptilolite zeolites, it is 5:1. Clino natural zeolites exhibit strong stability and resistance to decomposition in
weak acid environments, unlike synthetic zeolites. The more acid-resistant silica component in the structure of natural
zeolites contributes to their stability. Clino natural zeolites are widely used in agriculture as soil conditioners and feed
additives.
In 1948, Richard Barrer first synthesized a zeolite without a natural counterpart. Around the same time, Milton also
successfully produced the first batch of materials without natural counterparts, such as Zeolite A. Since then, new natural
zeolites are continuously being discovered, and numerous laboratories worldwide are continuously developing new
synthetic zeolites.
In summary, both synthetic and natural zeolites have their pros and cons, and the choice depends on specific application
requirements. Synthetic zeolites offer higher controllability and performance advantages, suitable for highly customized
environments, but with higher production costs. On the other hand, natural zeolites have lower costs and natural
sustainability but may perform less favorably than synthetic zeolites in some applications. Considering application
conditions, environmental factors, and economic benefits, selecting the appropriate type of zeolite can help achieve
optimal performance and cost-effectiveness.





